The sensor is inserted by an Eversense Inserter in the upper arm and continuously measures glucose for up to 6 months.
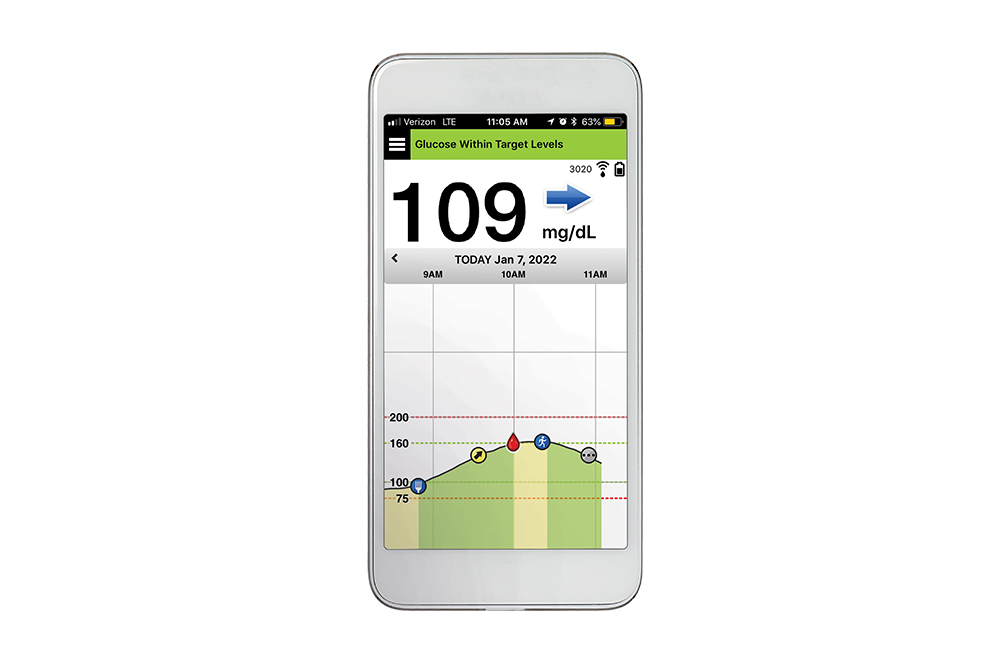
Eversense is the only long-term CGM system with 6 months of real-time glucose readings and only 2 sensors per year. Because you have life to manage, not just diabetes.
Apple has implemented two new features in iOS 17 that will affect your experience with the Eversense CGM System.
StandBy Mode, when enabled, activates the iPhone lock screen when the phone is charging and on its side. StandBy mode affects the Eversense App's ability to provide alerts in all the ways you expect. If StandBy mode is used, be sure you have turned on Show Notifications in Settings > StandBy. On-body vibe alerts from the transmitter are not impacted.
Assistive Access Mode, when enabled, provides a cognitive accessibility feature that will change the way you access the Eversense app and changes how alerts are presented when the app is not open. On-body vibe alerts from the transmitter are not impacted, however, this feature may result in brief, intermittent interference with the transmitter's Bluetooth connection to your phone. To download, install and create an Eversense Account, Assistive Access Mode must be turned off. Once the account is created, the Eversense App may be added to Assistive Access Mode.
You should disable automatic updates to your mobile device. Some operating system updates may result in a need for a supporting update to the Eversense Mobile App. Once we have verified compatibility of new operating systems with Eversense, our compatibility page will be updated.